HSP70: Disease Relevance
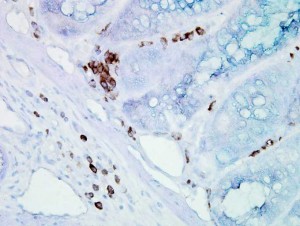
Immunohistochemical detection of Hsp70 in mouse inflamed colon tissue using Mouse Anti-Hsp70 Monoclonal Antibody, clone BB70.
Phenotypic analyses revealed that Hsp70-1 is found on the plasma membrane in 50 – 70% of colon, lung, pancreas, mammary, head and neck, lung and urogenital carcinomas 70, 199, 200. As demonstrated in a xenograft tumor mouse model, metastases exhibit an elevated Hsp70-1 membrane density compared to primary tumors after orthotopic injection of human tumor cells into immunodeficient animals 101, 201. Interestingly, the corresponding normal tissue of the mice was always found to be membrane Hsp70-1 negative 201 suggesting that membrane Hsp70-1 might facilitate metastases, support adherence of tumor cells to endothelial cells and organs, or might confer resistance to an unfavourable milieu during metastasis. In line with these findings overall survival of patients with lower rectal carcinomas and NSCLC exhibiting a membrane Hsp70-1 positive phenotype has been found to be significantly lower than that of their membrane Hsp70-1 negative counterparts 72. Apart from solid tumors, also bone marrow samples of patients suffering from acute (AML) and chronic (CML) myeloid leukemia are frequently membrane Hsp70-1 positive 202. Quantitative analysis revealed that approximately 15 – 20% of the total Hsp70-1 is present in tumor cell membranes 16. Tumor cells expressing Hsp70-1 on the plasma membrane are in general highly resistant to chemoradiotherapy most likely due to blockage of the NF-κB, JNK and ERK signaling pathways 203, 204. They can be eliminated by preactivated NK cells via granzyme B release in a perforin-independent, but Hsp70-1-dependent manner 95.
Despite its impact in carcinogenesis, HSP70s have also been shown to play critical roles in other pathological conditions. Overexpression of Hsp70-1 in patients with myelodysplastic syndromes (MDS) was found as being associated with poor prognosis 205. MDS comprise a group of clonal hematopoietic stem cell disorders characterized by varying degrees of pancytopenia and often a progression to acute myeloid leukemia 206. Enhanced plasma levels of Hsp70-1 were recently reported in patients with idiopathic inflammatory myopathy although the plasma levels did not correlate with clinicopathological parameters 207. Hsp70-1 upregulation was also reported for the peripheral lung tissues of chronic obstructive pulmonary disease (COPD) patients where it was closely related to COPD disease severity and smoking status 208. Of note, Hsp70-2 protein content from peripheral blood mononuclear cells (PBMCs) was significantly lower in multiple sclerosis (MS) patients with GG genotype compared to AA genotype, indicating an implication of the G allele of HSP70-2 gene (HSPA1B) polymorphism in the development of MS 209. A single nucleotide polymorphism in HSP70-2 has been shown to be associated with a severe clinical course in Crohn’s disease (CD). A recent study analyzed associations between the Hsp70-2 polymorphism and the clinical courses of CD in the Chinese population. Herein, the allele A of the PstI polymorphism was identified as the association between CD and HSP70-2 gene in this population 210. In patients with heart failure (HF), plasma concentrations of Hsp70-1 increased gradually with the progression of HF stages rendering Hsp70-1 a potential screening biomarker for early diagnosis of HF 211. This finding was extended by a further study identifying elevated extracellular Hsp70-1 levels as an independent prognostic marker of mortality in HF patients 212.
A growing body of evidence now indicates that the level and localization of Hsp70-5 (Grp78) is altered in different models of neurodegenerative disorders (ND) including Parkinson’s disease, Alzheimer’s disease and progressive retinal degeneration 213,214,215. These disorders are characterized by activation of the unfolded protein response (UPR) as a result of ER stress and modified expression of Hsp70-5 (Grp78), the ER homolog of Hsp70 and a key UPR mediator 216. Consequently, cell death pathways are activated involving a subsequent crosstalk between the ER and mitochondria leading to apoptosis 217. ER stress is therefore considered to be a common mediator of apoptosis in neurodegenerative disorders. The existing literature clearly indicates an age-dependent reduction of the Hsp70-5 expression and activation together with a modification of the ER structure in ND models 218, 219. This raises the question of whether the loss of Hsp70-5 function could be a predisposing factor for many age-associated ND including Parkinson’s disease, Alzheimer’s disease and age-related macular degeneration.
Hsp70-1 is present in the peripheral circulation of healthy nonpregnant and pregnant individuals. In normal pregnancy, circulating Hsp70-1 levels are decreased, and show a positive correlation with gestational age and an inverse correlation with maternal age 220. In contrast, elevated circulating Hsp70-1 concentrations are associated with an increased risk of several pregnancy complications. In preeclampsia, serum Hsp70-1 levels are increased reflecting systemic inflammation, oxidative stress and hepatocellular injury 221, 222. Moreover, serum Hsp70-1 levels are significantly higher in patients with the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome) than in severely preeclamptic patients without HELLP syndrome 223. In HELLP syndrome, elevated serum Hsp70-1 levels are indicative for tissue damage (hemolysis and hepatocellular injury) and disease severity 224.
In prokaryotes, DnaK has been shown as being required for survival of bacteria under stressful conditions 225. Consistent with this notion, mutants of dnaK from S. aureus show reduced viability and susceptibility to stress accompanied by an increased sensitivity towards oxacillin and methicillin in normally resistant S. aureus strains 226. Moreover, dnaK or dnaJ mutations in E. coli have been reported to sensitize the cells to fluoroquinolones 227. Since these chaperones are also required for the invasion of epithelial cells by Salmonella enerica and the survival of Listeria monocytogenes in macrophages 228, 229, Hsp70-1/DnaK and its co-chaperones can be considered as potential drug targets that would sensitize prokaryotes to stress. It is interesting to note that in mammalian cells infected with a virulent form of S. choleraesuis, the levels of Hsp70-1 correlated with an increase in TNF-induced cell death 230 highlighting its role in the host’s immune responses. More recently, Hsp70-peptide complexes (Hsp70.PC-F) extracted from fusions of dendritic cells (DCs) and radiation-enriched tumor cells were used to treat mice with pre-existing lung metastases 231. Immunization of mice with the Hsp70.PC-F vaccine resulted in a T cell-mediated immune response, including the induction of effector T cells capable of targeting radioresistant tumor cells. The combination of chaperone vaccine with radiotherapy inhibited the growth of primary tumors and the number of tumor cells metastasizing to lung suggesting that Hsp70.PC-F vaccine enable the induction of specific immunity to radioresistant populations of mammary tumor cells by complementing radiotherapy, leading to synergistic killing.231