HSP70: Mechanisms & Interactions
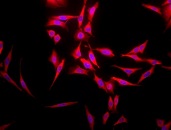
Immunofluorescent detection of Hsp70 using rabbit anti-Hsp70 polyclonal antibody in heat shocked HeLa cells.
Upon a variety of stresses, Hsp70-1 expression is rapidly induced via mitogen-activated protein kinase / extracellular signal-regulated kinase (MAPK/ERK) and c-Jun N-terminal kinase (JNK) signaling pathways culminating in the activation of HSFs 143,144,145. The molecular functions of Hsp70 in cancer cells are far from being understood but one aspect relies on interaction with multiple partners in the signaling pathways of apoptosis and senescence. Hsp70-1 has been found to interact with the mammalian MAPKKK apoptosis signal-regulated kinase 1 (Ask-1), JNK and p38MAPK leading to blockage of stress-induced cell death 146,147,148,149. In conjuction with the co-chaperone CHIP, a member of the E3 ubiquitin ligase, Hsp70-1 was found to promote proteasomal degradation of Ask-1 and to block TNF-mediated apoptosis through forming a ternary complex of Hsp70-1/CHIP/Ask-1 150. Notably, CHIP has been shown to downregulate the receptor tyrosine kinase c-Met critically involved in epithelial to mesenchymal transition (EMT), a crucial process in tumor invasiveness and metastasis 151, 152. Hsp70-1 also plays a pivotal role in TGF-β-mediated EMT by decreasing phosphorylation of the receptor-dependent TGF-β signaling transducer Smad-2 153.
Hsp70-1 is crucially involved in inhibiting programmed cell death because it binds directly to apoptosis protease-activating factor 1 (Apaf-1) and blocks recruitment of procaspase 9 to the mitochondrial apoptosome 154. Hsp70-1 was also found to sufficiently inhibit caspase 3-mediated apoptosis and to exhibit regulatory functions to JNK and ERK phosphorylation in hyperosmolarity-induced apoptosis 155. In addition, Hsp70-1 depletion triggered a p53-independent senescence program through inhibitory phosphorylation and downregulation of the cell cycle kinase Cdc-2 demonstrating the suppressive role of Hsp70 in senescence 87. A further binding target of Hsp70-1 has been identified as being the mitochondrial intermembranal flavoprotein apoptosis-inducing factor (AIF) which triggers chromatin condensation in a caspase-independent manner 156. These data clearly indicate that Hsp70-1 can inhibit apoptosis by interfering with extramitochondrial targets such as AIF thereby preventing AIF-induced chromatin condensation 156.
There is a wealth of evidence indicating the crucial contribution of Hsp70 to anoikis and amorphosis, apoptotic processes occurring to cells that lose their contact to the extracellular matrix (ECM) during cancer metastasis. In this context, Hsp70-1 has been reported to affect activity of c-Met 151, the major survival kinase Akt 157 and focal adhesion kinase (Fak) 158. Moreover, Hsp70 promotes chemoresistance of ovarian cancer cells by inhibiting Bax mitochondrial translocation 159 and stabilizes the metastasis-promoting protein Wiskott-Aldrich syndrome protein family member 3 (Wasf-3) 160. Hsp70 obviously plays an eminent role in the modulation of the ECM, since the Hsp70 regulator Bag-3 has recently been identified as interaction partner of MMP-2 161. The latter has been reported as being directly activated by Hsp70 complexed to Hsp90 and Hsp70/Hsp90 organizing protein (Hop) 162. In the monocytic cell line U937, extracellular Hsp70-1 has been shown to increase proMMP-9 secretion and activate MMP-9 expression via NF-κB and AP-1 163 highlighting the crucial role of Hsp70 in tumor invasion and metastasis.
Recent studies indicate an involvement of Hsp70 and Hsp90 in the recognition of pathogen-associated molecular patterns (PAMPs) by binding to Toll-like receptor 4 (TLR-4) within lipid rafts 164, 165. Since extracellular residing Hsp70 acts as a danger signal for the immune system 166, this stress protein has been added to the list of ‘alarmins’. Endogenous alarmins and exogenous PAMPs both comprise the group of danger-associated molecular patterns (DAMPs) 167. Human recombinant Hsp70, added exogenously to cells stimulates the production of proinflammatory cytokines (TNF, IL-1β, IL-6) and nitric oxide by antigen presenting cells (APCs) 84, 86, 168. Extracellular Hsp70-1 has also been found to induce IL-8 production in human bronchial epithelial cells 169. In vitro co-culturing of colon tumor cell spheroids with normal cells caused a significant tumor grade-dependent increase in IL-6 production thereby altering Hsp70-1 expression 170. IL-6 and IL-8 function as key players in carcinogenesis as they are required for initiation of tumor-associated inflammation and neovascularization 171, 172. From these findings it can be concluded that Hsp70 is able to enhance the impact of tumorigenic mediators in the tumor microenvironment.
Scavenger receptors (e.g. SREC-1, LOX-1, FEEL-1), the collagen/thrombospondin receptor CD35 and C-type lectin receptors (alpha 2 macroglobulin receptor CD91) on APCs have been found to facilitate the uptake of extracellular Hsp70 complexed to immunogenic peptides 173,174,175,176. Hsp70-chaperoned peptides are internalized via receptor-mediated endocytosis followed by cross-presentation to CD8+ T cells as well as initiation of innate immune responses 177, 178. Further ligands of these receptors are high mobility group box 1 (HMGB1), PAMPs and LPS 179.