HSP70: Drug Discovery
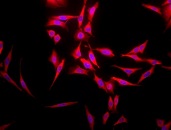
Immunofluorescent detection of Hsp70 using mouse anti-HSP70 monoclonal antibody, clone 3A3 in HeLa cells.
A promising approach in cancer therapy also might be targeting membrane-bound Hsp70-1. A membrane Hsp70-1 positive tumor phenotype has been found to be associated with a significantly decreased overall survival in tumor patients and could therefore serve as a negative prognostic marker 72. As already mentioned, Hsp70-1 serves as tumor-specific target structures for the recognition by NK cells that have been preactivated with low-dose IL-2 and the 14-mer Hsp70-1 peptide TKD 98,99,100,101. In a clinical phase I trial feasibility, safety and tolerability of ex vivo TKD/IL-2 stimulated autologous NK cells has been demonstrated in patients with metastasized colorectal and non-small lung cell carcinomas (NSCLC) 239. Based on these findings, a proof-of-concept phase II randomized clinical trial has been initiated to test the efficacy of ex vivo stimulated autologous NK cells in NSCLC patients following radio/chemotherapy. Since an Hsp70-1 membrane-positive tumor phenotype is associated with decreased patient`s overall survival, only patients with Hsp70 membrane positive tumors have been enrolled.
Due to their specific functions, tumor-derived HSPs have been used as promising tumor immunotherapy strategy. It has been shown previously that HSP preparations elicit immunity only against the tumors from which the derived 240. With regard to HSP70s, the immunogenicity of Hsp70 preparations isolated from tumors was found to derive, not from Hsp70 per se, but from associated peptides since the dissociation of peptides from Hsp70 abolished immunogenicity 241. This effect was achieved by utilizing the biochemical properties of both, HSP70 and HSP90 family members. Binding of HSP70 to ATP abolished its affinity for the client proteins whereas binding to ADP stabilized formation of HSP70 peptide complexes 35, 242. From these findings it became apparent that HSP peptide complexes (HSP.PCs) are the immunogenic entities eliciting antitumor immune responses. Thus, HSP70-based vaccines have been prepared and showed as being effective in limiting tumor growth 243. Clinical trials to test the effectiveness of this approach are ongoing 244, 245.
Animal studies as well as clinical trials demonstrated the efficacy, safety and feasibility of HSP-based tumor vaccines. The most studied tumor vaccine was the autologous tumor-derived HSP gp96 peptide complex vaccine HSPPC-96 (Vitespen) 240, 246, 247, 248. Unfortunately, Vitespen failed to show broad activity in randomized clinical trials despite encouraging results in selected patients (reviewed by Reitsma and Combest 245). The group of Giorgio Parmiani convincingly demonstrated that HSP70 (Hsp70-1 plus Hsp70-8) purified from human melanoma can activate T cells recognizing melanoma differentiation antigens in an antigen- and HLA class I-dependent fashion 249. The capacity to activate class I-restricted, antitumor T cells as well as APCs, together with the finding that the HSP70 chaperoned peptide repertoire includes melanoma-shared epitopes, highlighted the use of HSP70-based cancer vaccine in patients. However, insufficient immunogenicity and yield of patient tumor tissue limit the efficacy of the HSP-based vaccines.
In order to improve the effectiveness of HSP70-based cancer vaccines, Enomoto and colleagues concentrated on optimizing the peptide loading in an HSP70-based vaccine involving tumor/DC fusion and targeting breast cancer (Hsp70.PC-F or Hsp70 peptide complex from tumor/DC fusion cells). The rationale behind the Hsp70.PC-F approach is that fusion of tumor cells to DCs leads to optimal antigen processing by professional APC components and that the vaccine will contain a cross-section of tumor antigens whithout the need of further characterization 250. The Hsp70.PC-F vaccine also contained Hsp90 crucially involved in tumor regression 250. The peptide association could be improved further by rapid immunoprecipitation of Hsp70 and subsequent elution of the Hsp70 complexes leading to an Hsp70 vaccine with superior immunogenicity compared with standard affinity chromatography approaches 250, 251. In an attempt to develop a common Hsp70 vaccine, Hsp70.PC-F was extracted from DCs fused to patient-derived ovarian cancer cells or established human breast cancer cells, and their properties as tumor vaccines were examined 251. As demonstrated in this study, Hsp70.PC-F prepared from fusions of breast cancer cells and pooled human DCs stimulated T cell proliferation and enhanced tumor cell killing when compared to those induced by Hsp70.PC derived from tumor cells. Enhanced immunogenicity of Hsp70.PC-F was associated with improved composition of the vaccine, including increased content of tumor antigens and their processed intermediates, and the detection of other HSPs such as HSP90s and HSP110s. Thus, these might represent the basis for an alternative approach in the preparation of HSP-based vaccines using DC/tumor fusion technology and gentle and rapid isolation of HSP peptide complexes. Notably, this technique has the capacity to target individual tumor cell populations such as cancer stem cells (CSCs) 244. In ovarian carcinoma, CD44 has been identified as a surface marker for CSCs 252. T cells induced by DC-CSC fusion vaccine were found to selectively kill ovarian CSCs and radiation-resistant cells enriched in CSCs 252. Studies analyzing the efficaciousness of Hsp70.PC-F derived from DC-CSC fusion vaccines in targeting the CSC subpopulation are underway.