HSP70: Isoforms
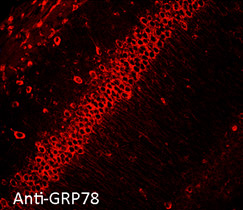
Immunofluorescent detection of Grp78 (Hsp70-5) in formaldehyde-fixed paraffin embedded sections of mouse hippocampal tissue, using Anti-Grp78 polyclonal antibody at 1:100
The human HSP70 family comprises 13 gene products differing from each other by expression level, subcellular location and amino acid constitution (Table 2). The major stress-inducible HSP70s comprise Hsp70-1 (HspA1A) and Hsp70-2 (HspA1B), collectively termed Hsp70 or Hsp70-1. The basal mRNA expression of HSPA1A/B varies in most tissues exceeding the expression levels of the other Hsp70 isoforms in humans 23.
Hsp70-1t (HspA1L) is a constitutively expressed, non-inducible cytosolic protein with high abundance in testis showing a ≈90% identity to Hsp70-1.
Hsp70.2 (HspA2) refers to the testis-specific HSP70 member. It is highly expressed not only in testis but also in brain and shows a ≈85% homology to Hsp70-1.Hsp70.2 has been suggested to play a pivotal role in spermatogenesis and meiosis 24, 25.
Hsp70-5 (HspA5, BiP, Grp78) is constitutively expressed in the ER facilitating transport and folding of nascent polypeptides into the ER lumen.
Hsp70-6 (HspA6) is a further heat-inducible HSP70 family member highly homologous to Hsp70-1 whose expression is induced after severe stress insults only 26.
HSPA7 has long been considered as being a pseudogene which is transcribed in response to stress, but recent observations suggest a high homology to HSPA6 19.
Hsp70-8 (HspA8, Hsc70, Hsp73) is the cognate HSP70 family member showing a 86% identity to Hsp70-1 with essential housekeeping functions such as folding and transport of polypeptides across intracellular membranes 27.
Hsp70-9 (HSPA9) is located to mitochondria and shows a 52% identity to stress-inducible Hsp70-1 although it is not induced upon stress. It bears a 42 amino acid target signal responsible for delivering Hsp70-9 to the mitochondrial lumen 28.
Hsp70-13 (HspA13, Stch) represents the microsome-associated member of the HSP70 family and is constitutively expressed in all human cell types 29.
Hsp70-14 (HspA14, Hsp70L1) is a novel HSP derived from human dendritic cells (DCs), with potent adjuvant effects polarizing responses toward Th1 30.
Less information is available for Hsp70-12A (HspA12A) and Hsp70-12B (HspA12B) representing more distally related members of the HSP70 family (e.g. Hsp70-12A is widely expressed with highest levels found in brain, kidney and muscle 31). The HSPA12A gene is conserved in human, chimpanzee, Rhesus monkey, dog, cow, mouse, and rat. Hsp70-12A and Hsp70-12B conserve only sparse similarity to the ATP-binding domain of typical human HSP70 proteins, and are marginally more similar to prokaryotic DnaK 32.
A comprehensive characterization of the extended family of HSP70 genes and their proteins based on genomic, structural, and evolutionary analyses identified HSPA12 genes only in vertebrates such as fish (Danio), amphibians (Xenopus), birds (chicken), and mammals (human and mouse), suggesting that on the one hand the various HSPA12 genes evolved at an early stage in vertebrate evolution and on the other hand versions of the gene that diverged earlier do not conserve significant similarity to current human homologs 19. As given in this analysis, zebrafish (Danio rerio) expresses three diverged copies of the gene (similarities 97–99%) that were quite diverged from the human and amphibian sequences (similarities in the range 25–32%). Chicken had only one copy which was most similar to the human HSPA12B (92% similarity), whereas two copies of this gene were found in mammals and amphibians. The amphibian and mammalian genes obviously originated from a unique duplication event occurring in the tetrapoda lineage before the radiation of the amphibian and mammalian lineages. Among the amphibians and mammals examined, the two gene copies have an average similarity of 63%. Each paralog is quite conserved, with similarities between mammals and amphibians of approx. 82% for the HSPA12A paralog, and of 71–73% for the HSPA12B paralog, whereas between human and mouse similarities are 97% and 94%, respectively. The phylogenetic relations of the vertebrate lineages imply that one of the two copies of the gene has been lost from the chicken genome. Interestingly, the authors identified a non-annotated sequence in the chicken genome encoding a protein with 56.5% identity to a segment of the annotated chicken HSPA12 gene, most likely a remnant of HSPA12A.
The same study revealed that the human Hsp70-6 and Hsp70-7 sequences do not significantly cluster with any other animal protein. This result is consistent with an early separation of Hsp70-6/7 and the common progenitor of Hsp70-8, Hsp70.2 (HspA2) and Hsp70-1, presumably preceding vertebrate evolution. The presence of Hsp70-8 sequences from fish and amphibians and the lack of a sequence from fish in the HspA1/2 lineages implies that Hsp70-1 and Hsp70.2 (HspA2) originated from Hsp70-8 early in tetrapoda evolution, with the exception of fish, although homologs of these genes are occasionally found in zebrafish, Danio rerio 19.