HSP70: Localization
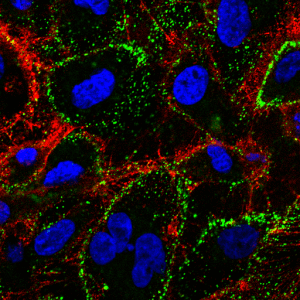
Immunofluorescent detection of cell membrane embedded Hsp70 using Mouse anti-HSP70 Antibody, clone 1H11 (green) in non-permeabilized HCT116 cells.
Apart from their intracellular localization, Hightower and Guidon reported on an ER/Golgi-independent release of Hsp70-1 from viable cells with intact cell membranes already in the late 1980s 61. The mechanisms of HSP export are still controversially discussed since cytosolic HSPs lack a consensus signal for secretion. However, apart from Hsp70-1, other molecules lacking a secretory signal such as IL-1α, IL-1β, and high mobility group box 1 (HMBG-1) are also found outside of cells 62, 63. Hsp70-1 also has been found to be located on the cell surface although lacking a transmembrane domain 64. Membrane Hsp70-1 might help to maintain stability of tumor cells and thus might protect tumors from lethal damage induced by environmental stress 65, 66. The pioneering work of the group of Antonio De Maio has demonstrated an interaction of members of the HSP70 family with artificial membranes containing phosphatidylserine (PS) 15, 67,68,69. An interaction of Hsp70-1 with the sphingolipid globoyltriaosylceramide (Gb3) in the plasma membrane of non-stressed human gastrointestinal stromal tumors has also been reported 16. Gb3 is found in cholesterol-rich microdomains, also termed as lipid rafts, which serve as signal transduction platforms. Following irradiation or hypoxia-induced stress, Hsp70-1 was found to be associated predominantly with PS outside of lipid rafts in the plasma membrane of tumor cells 17. These data indicate that environmental stress might result in a re-organization of the lipid bilayer and might modulate the interaction of Hsp70-1 with lipid components. Surprisingly, only tumors but not the corresponding normal tissues were found as being membrane Hsp70-1 positive using the IgG1 mouse monoclonal antibody cmHsp70.1 70, 71. A membrane Hsp70-1 positive tumor phenotype has been found to be associated with a significantly decreased overall survival in tumor patients. Therefore, the expression of this molecule could serve as a negative prognostic marker 72. In contrast, other Hsp70-specific antibodies failed to bind to membrane Hsp70-1 on viable tumor cells 18. The discovery that neither high/low salt concentrations nor changes in the pH were able to release Hsp70-1 from the plasma membrane of tumor cells 15, 16 led to the hypothesis that in tumor cells Hsp70-1 is an integral membrane protein which can associate with raft (Gb3) and non-raft (PS) lipid components. It is worth mentioning that therapeutic interventions such as radiochemotherapy upregulates the cell surface expression of Hsp70-1 selectively on tumor cells 73,74,75,76 rendering integral membrane Hsp70-1 a tumor-specific recognition structure for therapeutic interventions.
It has been shown previously that viable Hsp70-1 membrane-positive tumors release Hsp70-1 surface-expressing lipid vesicles like the tumors from which they originate 13, 14, 77. Other export mechanisms might include the normal lysosomal/endosomal pathway 78, 79 or cell lysis 80. According to Multhoff and Hightower (2011), the appearance of Hsp70-1/peptide complexes in the circulation most likely results from secretion by necrotic tissues 81.