HSP70: Function
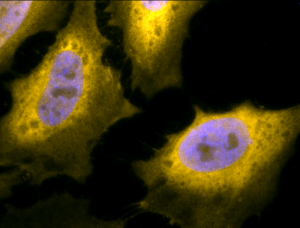
Immunofluorescent detection of Hsp70 using Chicken Anti-Hsp70 Polyclonal Antibody in heat shocked HeLa cells.
Moreover, HSPs serve as tumor-specific target structures for the recognition by activated NK cells. Even in the absence of immunogenic peptides, Hsp70-1 or a peptide derived thereof in combination with proinflammatory cytokines such as IL-2 and IL-15 has the capacity to stimulate the cytolytic activity of NK cells against membrane Hsp70-1 positive tumor cells 93, 94. The mechanism of tumor cell killing has been identified as perforin-independent granzyme B-mediated apoptosis 95. Granzyme B derived from activated NK cells specifically binds to membrane Hsp70-1 on tumor cells, and following Hsp70-1 mediated endocytosis apoptosis is induced 95, 96. Hsp70-1 also has been detected on tumor-derived exosomes of membrane Hsp70-1 positive tumors 13. These data suggest that NK cells might be attracted to membrane Hsp70-1 positive tumors in vivo via the secretion of Hsp70-1 surface-positive exosomes. Incubation of NK cells with Hsp70-1 protein or a 14mer-peptide derived from the C-terminus of Hsp70-1 (termed TKD) is accompanied by an upregulation of activating receptors on NK cells such as CD94/NKG2C, NKG2D, NKp30, NKp44, and NKp46 95, 97. Hsp70-1 membrane-positive tumors are thus efficiently eliminated by NK cells that had been pre-stimulated with low dose IL-2 plus Hsp70-1 peptide 98. Adoptive transfer of these TKD-stimulated NK cells in tumor-bearing mice revealed identical results in vivo 99,100,101. It is known that IL-2-activated NK cells are able to induce regression of established lung and liver tumors 102,103,104,105. Recently, a specific migratory capacity of NK cells towards Hsp70 postive tumor cells and supernatants derived thereof could be demonstrated. The same effect could be observed for the Hsp70-1 peptide TKD 106 implying that killing of Hsp70-1 positive tumors in vivo might be related to an enhanced migratory and cytolytic capacity of preactivated NK cells.